Abstract
Background: Chimeric antigen receptor T cells (CARs) targeting CD22 can provide a therapeutic avenue for patients who progress or develop antigen loss after receiving CD19-directed therapies for hematologic malignancies. A prior Phase 1 trial of CD22 CARs in pediatric patients with relapsed/refractory (r/r) B cell acute lymphoblastic leukemia (ALL) demonstrated a 73% complete response (CR) rate with a median remission duration of 6 months.1 We hypothesized that similar activity would be seen in a wider array of patients, including adults. Here, we report our clinical experience with CD22 CARs in a mixed cohort of pediatric and adult patients with r/r ALL.
Methods: This Phase Ib trial conducted at Stanford University was designed to test the safety and feasibility of a CD22-targeting CAR T cell product in pediatric and adult patients with r/r ALL. All patients were screened for CD19 and CD22 expression prior to enrollment. The CAR was manufactured using an automated CliniMACS Prodigy (Miltenyi Biotec, Bergisch Gladbach, Germany) lentiviral transduction system2 that has been previously used to successfully create single and dual-targeted CARs.3,4 Sixteen patients received lymphodepletion with fludarabine (30 mg/m2/day IV on Days -5, -4, and -3) and cyclophosphamide (500mg/m2/day on Days -5, -4, and -3) followed by fresh or cryopreserved CAR T cell infusion after a 7-9 day production time. Patients were prospectively monitored at predefined intervals for disease response and correlative assessments.
Results: Eighteen patients were enrolled, though 2 pediatric patients died before receiving therapy. Patients were a median age of 23 years old (range 2-62) and had received a median of 5 prior lines of therapy (range 3-8). Thirteen patients (72%) were previously exposed to CD19-targeting agents; 9 (50%) had received CAR T cells and 11 (61%) had received blinatumomab. Four patients (22%) had also received inotuzumab. At enrollment, 8 patients (44%) had CD19 low/negative disease (defined as <90% surface expression detected by flow cytometry), and all patients expressed CD22 as per trial eligibility criteria.
Sixteen of 18 patients were infused on trial. Cytokine release syndrome (CRS) occurred in 11 patients (72%); however, 10 (92%) had ≤ Grade 2 CRS and only 1 patient had Grade 3 CRS. Immune effector cell-associated neurotoxicity syndrome (ICANS) occurred in 1 patient (Grade 4). Three of 16 patients (19%) developed CAR-related hemophagocytic lymphohistiocytosis (car-HLH), as seen previously with this product.5 One patient who developed car-HLH died from infection.
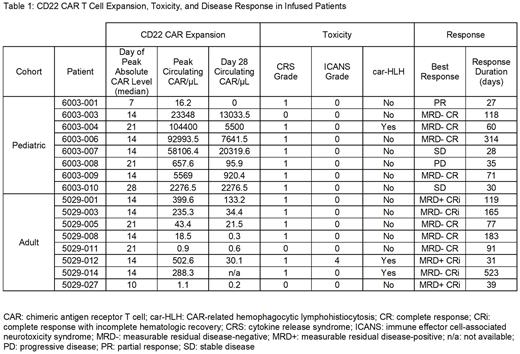
Of the 16 patients infused with CD22 CARs, 12 (75%) achieved a CR or CR with incomplete hematologic recovery (CRi) and 9 (56%) were negative for measurable residual disease (MRD) by flow cytometry (Table 1). In the adult cohort (n = 8) there was a 100% rate of CR/CRi with 5 patients achieving MRD-negativity (63%). Four of 8 pediatric patients (50%) achieved CR, all MRD-negative. In the entire cohort during the first 28 days post-infusion, the median peak CAR absolute count was 451 cells/µL, with a median Day 28 absolute count of 96 cells/µL. Pediatric patients had substantially greater levels of both peak [mean: 35,921 cells/µL (range 16 - 104,400)] and Day 28 [mean: 6223 cells/µL (range 0 - 20,320)] CAR levels compared to adults [mean peak level: 451 cells/µL (range 1-503), mean Day 28 level: 96 (range 0.2-133)]. The median day of peak CAR expansion was Day +14. The median duration of response (either until relapse or next therapy) was 105 days in adults, 47.5 days in pediatrics, and 74 days overall. Twelve patients relapsed after CD22 CAR and overall survival at one year was 50% (8/16).
Conclusion: The results of our Phase Ib trial recapitulate previous findings with CD22 CARs and demonstrate that this CAR maintains its safety and feasibility profile in adults and children with heavily pre-treated ALL. The CD22 CAR resulted in high rates of CR and MRD-negativity independent of prior CD19 CAR and/or blinatumomab exposure. CRS was common but reversible, and the incidence and grades of CRS, ICANS and car-HLH were consistent with long-term follow up data from CD22 CAR studies in pediatric populations.6 CD22 CARs thus present a promising option for both pediatric and adult patients with r/r ALL.
Disclosures
Frank:Adaptive Biotechnologies: Consultancy, Honoraria, Research Funding; Kite/Gilead: Honoraria, Research Funding; Roche/Genentech - Wife: Current equity holder in private company, Current holder of stock options in a privately-held company; Allogene Therapeutics: Research Funding. Feldman:Fresh Wind Bio: Consultancy; Alaunos: Consultancy; Syncopation Life Sciences: Honoraria, Other: Sponsored Research. Miklos:Janssen: Consultancy, Honoraria; Pharmacyclics: Patents & Royalties: cGVHD Ibrutinib patent ; Fosun Kite: Consultancy, Honoraria; Adaptive Biotech: Consultancy; Kite, a Gilead Company: Research Funding; Bristol Meyers Squibb: Consultancy; Novartis: Consultancy; Allogene: Research Funding. Mackall:Syncopation: Consultancy, Divested equity in a private or publicly-traded company in the past 24 months; Immatics: Consultancy; Mammoth: Divested equity in a private or publicly-traded company in the past 24 months; Medimmune Tech: Consultancy; Nektar: Consultancy; Ensoma: Divested equity in a private or publicly-traded company in the past 24 months; Link: Consultancy, Divested equity in a private or publicly-traded company in the past 24 months; Apricity: Consultancy, Divested equity in a private or publicly-traded company in the past 24 months; BMS: Consultancy; GSK: Consultancy; Lyell Pharmaceuticals: Consultancy, Divested equity in a private or publicly-traded company in the past 24 months. Muffly:Novartis: Research Funding; UpToDate: Consultancy, Honoraria; Adaptive: Honoraria; BMS: Research Funding; Adaptive: Honoraria, Research Funding; Jasper: Research Funding; Astellas: Consultancy, Research Funding; CTI Biopharma: Consultancy; Medexus: Consultancy; Kite: Consultancy, Research Funding; Pfizer: Consultancy; Amgen: Consultancy. Schultz:Novartis: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal